Your cart is empty
Start Shopping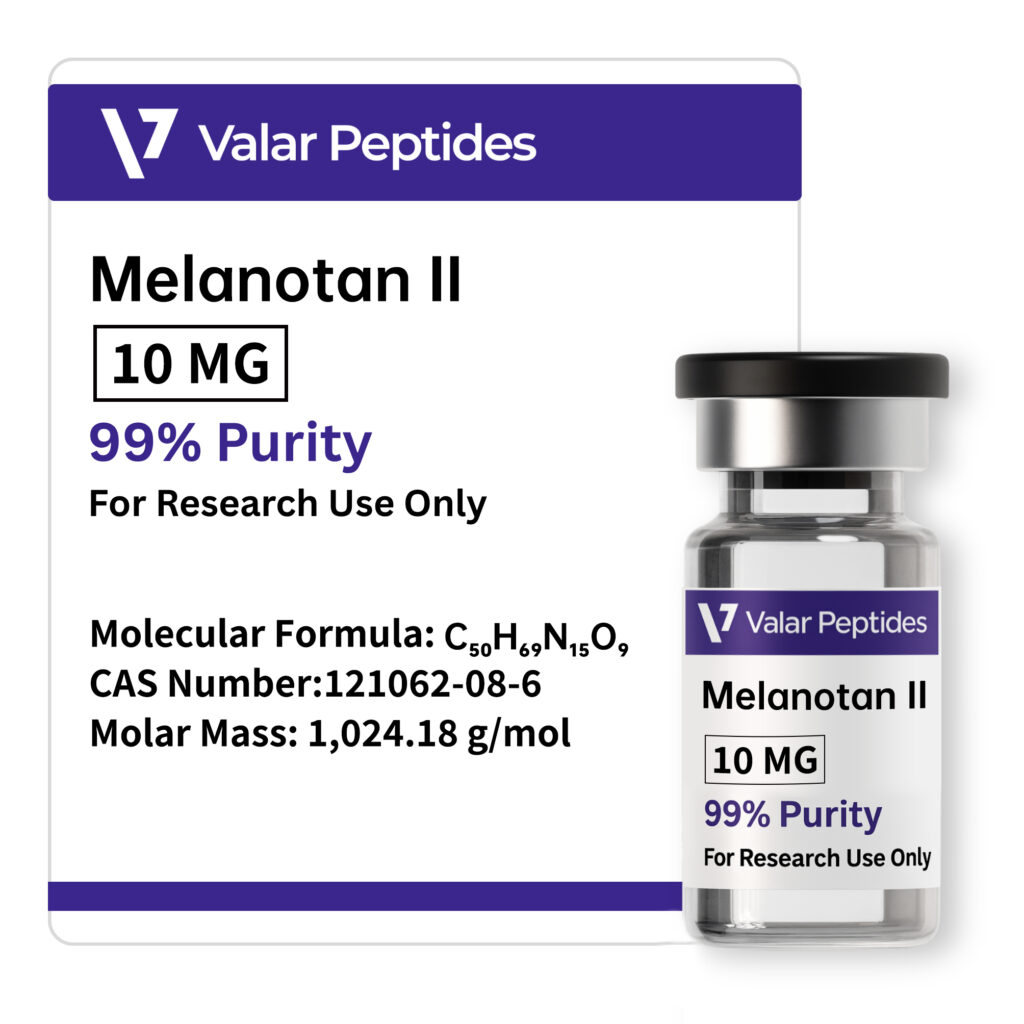
$40.00
This material is sold for laboratory research use only. Terms of sale apply. Not for human consumption, nor medical, veterinary, or household uses. Please familiarize yourself with our Terms & Conditions prior to ordering.
| Molecular Formula | C₅₀H₆₉N₁₅O₉ |
| CAS Number | 121062-08-6 |
| Molar Mass | 1024.18 g/mol |
| Amino Acid Sequence | Ac-Nle-c[Asp-His-D-Phe-Arg-Trp-Lys]-NH2 |
| PubChem CID | 92432 |
| Primary Research Area |
Energy Homeostasis Body Mass Regulation Appetite Suppression Skin Pigmentation (Sunless Tanning) Spontaneous Penile Erection & Sexual Stimulation |
| Purity | >99% |
| Research Summary | Description |
|---|---|
| Evaluation of melanotan-II, a superpotent cyclic melanotropic peptide in a pilot phase-I clinical study |
Summary: This pilot Phase 1 study aimed to evaluate the safety, tolerability, and melanogenic (tanning) effects of Melanotan II in healthy volunteers. While specific details on the tanning efficacy in this early pilot are limited in publicly available summaries, the study confirmed that Melanotan II could induce skin pigmentation. It also noted the occurrence of side effects, including nausea, flushing, and spontaneous erections in male subjects, which, while unintended, led to further research into its use for sexual dysfunction. Citation: Dorr, R. T., Lines, R., Levine, N., Brooks, C., Xiang, L., Hruby, V. J., & Hadley, M. E. (1996). Evaluation of melanotan-II, a superpotent cyclic melanotropic peptide in a pilot phase-I clinical study. Life Sciences, 58(20), 1777–1784. |
| Activation of the central melanocortin system chronically reduces body mass without the necessity of long-term caloric restriction |
Summary: This study demonstrated that chronic central administration of Melanotan II in rats led to a persistent reduction in body mass, even after food intake normalized following an initial transient anorexic response. Citation: Lucas, M., Zhang, M., & Bi, S. (2015). Activation of the central melanocortin system chronically reduces body mass without the necessity of long-term caloric restriction. Physiology & Behavior, 152(Pt A), 1-8. |
| Melanotan II: a possible cause of renal infarction: review of the literature and case report |
Summary: A case report by Lund et al. (2020) described a renal infarction likely attributed to Melanotan II use, suggesting possible direct toxic effects on renal parenchyma and thrombotic influences. The peptide's ability to overstimulate the sympathetic nervous system, leading to vasoconstriction, was proposed as a mechanism for renal injury. Citation: Lund, J., Thomsen, M., & Jensen, J. D. (2020). Melanotan II: a possible cause of renal infarction: review of the literature and case report. Clinical Kidney Journal, 13(2), 209-212. |
| Synthetic melanotropic peptide initiates erections in men with psychogenic erectile dysfunction: double-blind, placebo controlled crossover study. |
Summary: This early, small-scale human clinical trial (n=10) investigated Melanotan II's ability to induce erections in men with psychogenic erectile dysfunction. Participants received either Melanotan II (0.025 mg/kg subcutaneously) or a placebo. The study found that 8 out of 10 men treated with Melanotan II developed clinically apparent erections, with a significantly longer duration of rigidity compared to placebo. Transient side effects such as nausea, stretching, yawning, and decreased appetite were more frequently reported with Melanotan II. Citation: Wessells, H., Fuciarelli, K., Hansen, J., Hadley, M. E., Hruby, V. J., Dorr, R., & Levine, N. (1998). Synthetic melanotropic peptide initiates erections in men with psychogenic erectile dysfunction: double-blind, placebo controlled crossover study. The Journal of Urology, 160(2), 389–393. |
| Melanocortin receptor agonists in the treatment of male and female sexual dysfunctions: results from basic research and clinical studies. |
Summary: This review article provides a broader perspective on melanocortin receptor agonists, including Melanotan II and its metabolite bremelanotide, for sexual dysfunction. It summarizes the central mechanism by which these peptides induce sexual arousal and erection, specifically through MC3 and MC4 receptor activation in the brain. It discusses the success of bremelanotide (derived from Melanotan II research) in later clinical trials for female sexual interest/arousal disorder, highlighting how the core mechanism identified with Melanotan II was refined into a more selective and tolerable drug for a specific indication. Citation: Pfaus, J. G., & Sadowski, B. (2014). Melanocortin receptor agonists in the treatment of male and female sexual dysfunctions: results from basic research and clinical studies. Expert Opinion on Investigational Drugs, 23(11), 1477–1483. |
Summary: This pilot Phase 1 study aimed to evaluate the safety, tolerability, and melanogenic (tanning) effects of Melanotan II in healthy volunteers. While specific details on the tanning efficacy in this early pilot are limited in publicly available summaries, the study confirmed that Melanotan II could induce skin pigmentation. It also noted the occurrence of side effects, including nausea, flushing, and spontaneous erections in male subjects, which, while unintended, led to further research into its use for sexual dysfunction.
Citation:
Dorr, R. T., Lines, R., Levine, N., Brooks, C., Xiang, L., Hruby, V. J., & Hadley, M. E. (1996). Evaluation of melanotan-II, a superpotent cyclic melanotropic peptide in a pilot phase-I clinical study. Life Sciences, 58(20), 1777–1784.
Summary: This study demonstrated that chronic central administration of Melanotan II in rats led to a persistent reduction in body mass, even after food intake normalized following an initial transient anorexic response.
Citation: Lucas, M., Zhang, M., & Bi, S. (2015). Activation of the central melanocortin system chronically reduces body mass without the necessity of long-term caloric restriction. Physiology & Behavior, 152(Pt A), 1-8.
Summary: A case report by Lund et al. (2020) described a renal infarction likely attributed to Melanotan II use, suggesting possible direct toxic effects on renal parenchyma and thrombotic influences. The peptide's ability to overstimulate the sympathetic nervous system, leading to vasoconstriction, was proposed as a mechanism for renal injury.
Citation: Lund, J., Thomsen, M., & Jensen, J. D. (2020). Melanotan II: a possible cause of renal infarction: review of the literature and case report. Clinical Kidney Journal, 13(2), 209-212.
Summary: This early, small-scale human clinical trial (n=10) investigated Melanotan II's ability to induce erections in men with psychogenic erectile dysfunction. Participants received either Melanotan II (0.025 mg/kg subcutaneously) or a placebo. The study found that 8 out of 10 men treated with Melanotan II developed clinically apparent erections, with a significantly longer duration of rigidity compared to placebo. Transient side effects such as nausea, stretching, yawning, and decreased appetite were more frequently reported with Melanotan II.
Citation:
Wessells, H., Fuciarelli, K., Hansen, J., Hadley, M. E., Hruby, V. J., Dorr, R., & Levine, N. (1998). Synthetic melanotropic peptide initiates erections in men with psychogenic erectile dysfunction: double-blind, placebo controlled crossover study. The Journal of Urology, 160(2), 389–393.
Summary: This review article provides a broader perspective on melanocortin receptor agonists, including Melanotan II and its metabolite bremelanotide, for sexual dysfunction. It summarizes the central mechanism by which these peptides induce sexual arousal and erection, specifically through MC3 and MC4 receptor activation in the brain. It discusses the success of bremelanotide (derived from Melanotan II research) in later clinical trials for female sexual interest/arousal disorder, highlighting how the core mechanism identified with Melanotan II was refined into a more selective and tolerable drug for a specific indication.
Citation:
Pfaus, J. G., & Sadowski, B. (2014). Melanocortin receptor agonists in the treatment of male and female sexual dysfunctions: results from basic research and clinical studies. Expert Opinion on Investigational Drugs, 23(11), 1477–1483.
Your cart is empty
Start Shopping